World-first Australian clinical trial shows promise for suppressing progression of type 1 diabetes in those newly diagnosed

A JDRF-funded, world-first clinical trial has shown that a commonly prescribed rheumatoid arthritis drug (baricitinib) can preserve the body’s own insulin production and suppress the progression of type 1 diabetes (T1D) in those newly diagnosed with the condition.
This comes after three decades of research out of the St Vincent’s Institute (SVI) in Melbourne, led by Professors Thomas Kay and Helen Thomas, investigating the mechanisms in T1D that lead the immune system to attack beta cells.
The BANDIT (Baricitinib in New Onset Type 1 Diabetes) trial findings demonstrated that the once-daily administration of a drug called baricitinib over a 48-week period preserved beta cell function, reduced blood glucose fluctuations, and reduced the need for insulin in those who had been diagnosed within the past 100 days. The findings were today published in a leading medical journal, The New England Journal of Medicine.

“Our BANDIT trial has determined that baricitinib can preserve beta cell function and insulin production in people recently diagnosed with type 1 diabetes. This suggests that if given early enough baricitinib may allow people with type 1 diabetes to be significantly less dependent on insulin treatment.”
Professor Thomas Kay, Principal Investigator, BANDIT study
About baricitinib and its potential role in T1D
- In T1D, the immune system mistakenly attacks insulin-producing beta cells, meaning that those with well-established T1D can no longer produce their own insulin. However, in people who are newly diagnosed, a small number of beta cells can still be alive and produce insulin.
- Baricitinib is a drug which is currently used to treat rheumatoid arthritis and other conditions. It works by inhibiting a protein called Janus kinase (JAK) which is important in the immune system attack of beta cells in T1D.
- This suggests that inactivating JAK with baricitinib in those newly diagnosed may protect the remaining beta cells from immune attack, allowing people to keep producing their own insulin for longer. This would reduce their need to inject insulin.
About the trial
- The BANDIT trial was undertaken in Australia and recruited 91 people aged 10-30 years who were newly diagnosed with T1D in the previous 100 days.
- Participants were either provided baricitinib in tablet form, once daily for 48 weeks, or a placebo.
- The trial primarily investigated the level of C-peptide (a sign of insulin production) at 48 weeks.
- It also investigated the need for injected insulin, how well blood sugar levels were controlled via CGM (continuous glucose monitoring) and HbA1c (a marker of longer-term control of blood sugar levels).
The results
- This world-first study demonstrated that the administration of baricitinib led to a stabilising of C-peptide and hence sustained insulin production at 48 weeks.
- Baricitinib use also led to decreased insulin requirements, improved time in range, and improved blood glucose variability at 12 and 24 weeks compared to the placebo group.
- Baricitinib was also well tolerated, with minimal side effects.
What does this mean for the future of T1D research?
These breakthrough findings suggest that baricitinib may preserve beta cell function in those newly diagnosed and may represent a new therapeutic option for delaying T1D progression.
This indicates that baricitinib can be safely used to suppress the progression of T1D. More beta cell function should lead to better and easier blood glucose management and may in turn lower rates of long-term complications for those with T1D.
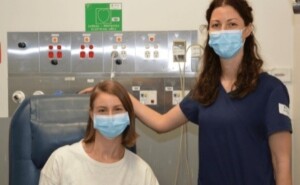
“It’s good to be part of this trial, to help researchers find new treatments for people with type 1 diabetes. Participating has also been really helpful in having access to closer monitoring and support, via the clinical trial team, in the early stages of my diagnosis”.
Lucy, first T1D participant signed up to the BANDIT trial with staff member.
This is an exciting first step and further work will be necessary to understand how this class of medication could be made available to people living with T1D.
Here’s a video explaining a little more about BANDIT:
JDRF Australia’s vision is for a world without T1D, and we work towards this by funding the most promising research aimed at finding cures and improving lives.
“JDRF focusses on funding research with the biggest potential for immediate impact for our community, and these results show an important step towards novel type 1 diabetes treatments. We’re proud to have supported a clinical trial that builds on many years of work led by the team at SVI – a demonstration of translational research in action with clear pathway to reduce burden of people living with T1D.”
Dr Dorota Pawlak, JDRF Australia Chief Scientific Officer
This was jointly funded by JDRF Australia, through our Type 1 Diabetes Clinical Research Network, and JDRF International.
Our research portfolio
Groundbreaking projects like these are only possible with support from our community. The future of 130,000 Australians living with T1D and the eight more diagnosed each day depends on it.
To get involved, donate here.