Continuous glucose monitors
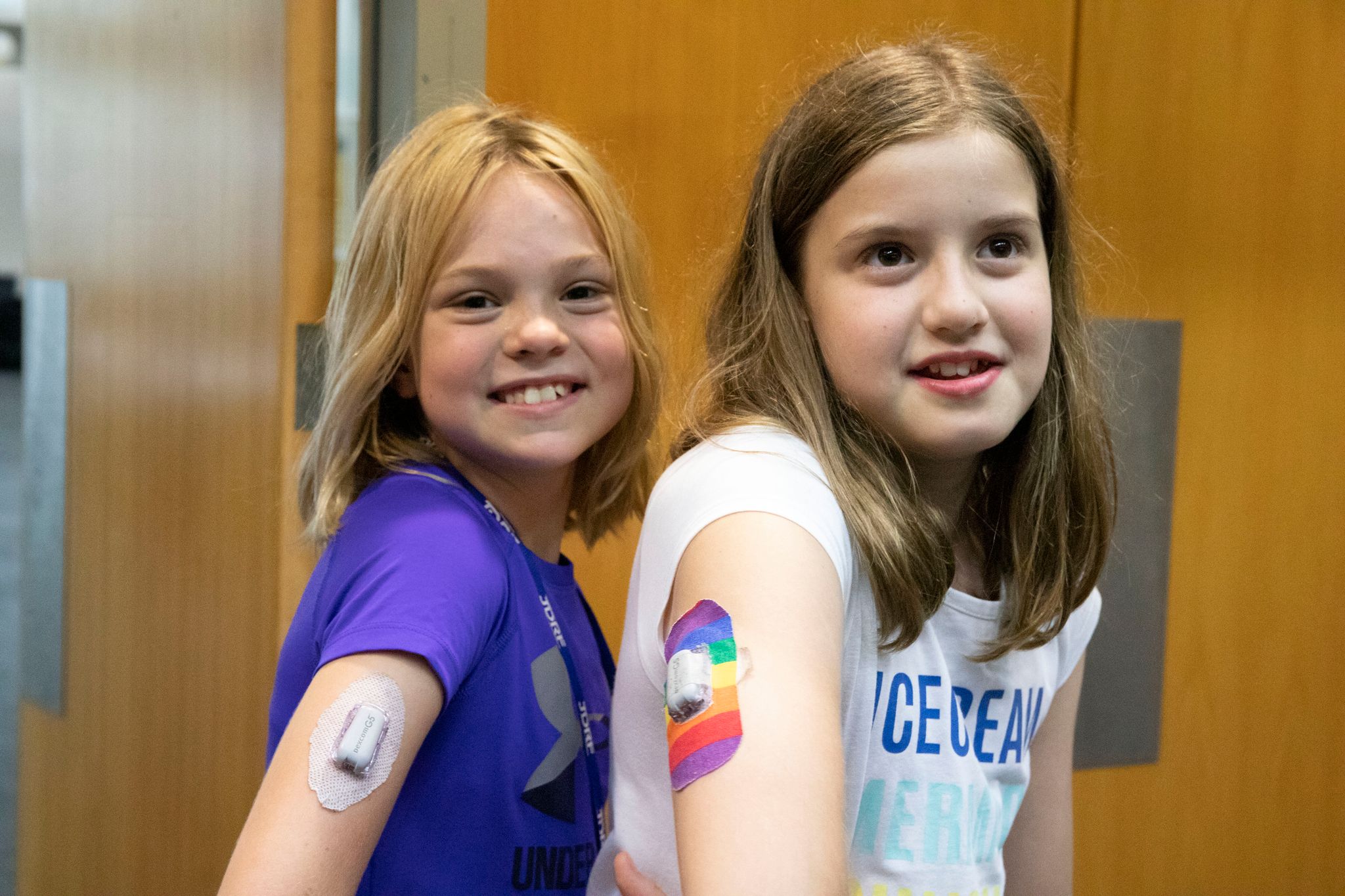
About continuous glucose monitors
A continuous glucose monitor (CGM) is a wearable device that continuously tracks glucose levels, day and night, allowing users to see patterns and trends. CGM units sound an alarm if blood glucose levels are changing rapidly, helping people avoid hypoglycaemia or hyperglycaemia.
Each system requires a sensor or a tiny electrode to be inserted under the skin. This is attached to either a transmitter or directly to the back of the upper arm. The electrode measures the level of glucose in the interstitial fluid in the tissue every 3 to 5 minutes. Depending which system is used, the sensor usually needs to be replaced every 6 to 14 days.
Real time CGMs
A real time CGM actively sends the glucose readings to a device, such as a mobile phone or insulin pump, and alarms can be set to alert when the sensor glucose level is too high or too low.
As measurements are taken every 3 to 5 minutes, the direction in which the glucose level is trending can be seen and some systems can alert up to 20 to 30 minutes before the sensor glucose is expected to be too high or too low, allowing some action to be taken beforehand. When used in conjunction with some insulin pumps, insulin delivery can either be stopped, decreased or increased based on the sensor glucose reading. This can have the added benefit of helping to prevent hypoglycaemia and hyperglycaemia with minimal input needed from the person living with T1D.
Read more about how hybrid closed-loop pumps work with CGMs.
Subsidised CGM access
As of 2022, CGMs have been subsidised for every Australian diagnosed with T1D. This means everyone living with the condition can access the technology if they wish to, with no barriers based on cost or other factors.
This outcome followed a long and winding road of advocacy, with incremental success along the way.
The subsidisation journey in a snapshot:
Initial CGM subsidisation
In May 2016, the Coalition made a pre-election commitment of $54m towards CGM reimbursement with no out-of-pocket costs for people with T1D under the age of 21. In June 2016 the ALP also made an election commitment of $79.7m for CGM reimbursement.
The CGM initiative was launched on 1 April 2017. In November 2018, it was extended to children and young people with conditions similar to T1D (such as neonatal-onset diabetes) that require insulin, women who are pregnant, planning pregnancy or immediately post-partum, and people with T1D aged 21 and older who have concessional status.
Expanded subsidisation and #AccessForAll
After years of continued campaigning, 2022 marked a $273.1 million bipartisan commitment on the 17th of April for CGMs to be available through the NDSS from 1 July 2022 for everyone, with a maximum co-payment of $32.50 per month only for those over 21 who did not otherwise have access.
The eligibility criteria to provide fully subsidised CGM products with no co-payment stays the same for those under 21, concession card holders and pregnant women.

I am interested in hearing more about…
